Across the English Channel a small but dedicated company called Diabeloop is pushing forward a closed-loop insulin delivery system.
Diabeloop was founded in 2015 and is based in Grenoble, France. Most members of staff are based here, though there is also an office in Paris. In November 2018, Diabeloop’s first medical device, DBLG1, was CE marked. This company is working on delivering the system in Europe (France and Germany at first) and later in North America.
Founder and CEO Eric Hunekerhas experience medical devices field at GE Healthcare, where he managed the international localization business to a ten-fold growth in sales. He has been the driver of the technology side of development.of Diabeloop’s solutions when Guillaume Charpentier co-founder was looking into transforming his medical project into a commerically available solution. Co-CEO Mark Julien looks after the financing and fund-raising.
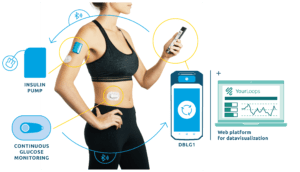
Stephanie Jegu works for the company, looking after brand & communications. This is the first time she’s worked in the medical field, having previously worked with companies outside of the healthcare sector. Jegu has had Type I diabetes for nearly a decade, so can easily add insights as to the benefit of the system. She explains, “DBLG1 is described as a ‘hybrid closed-loop insulin delivery system’. It’s ‘hybrid’ because although the insulin delivery is automated and personalized, the user still has to enter information, such ascarbs intake and physical activity for the system to be able to anticipate glucose variation and adapt delivery and/or recommendations. The system is designed to be easy to use and has a friendly interface.”
At the end of 2020, another device of Diabeloop, DBL-hu, obtained CE-mark. It targets highly unstable diabetes, which is very complex to manage and people who have it struggle to gain and maintain decent blood glucose balance. The clinical and metabolic consequences are debilitating and lead to severe progressive complications can develop. Diabeloop’s DBL-hu modular technological solution is designed to provide people with complex diabetes a more stable daily life, reduced psychological distress, reduced hypoglycemia and better overall diabetes control.
Robust trials
Diabeloop pursues a programme of clinical studies. Results for the DBL4K clinical study with 15 participating children are set to be published soon, and a study on adolescents, (including unannounced meal functionality) should start in 2021.
Any closed loop system involves a continuous glucose monitor (CGM) working with an insulin delivery device via an algorithm). Diabeloop currently works with ViCentra’s Kaleido insulin pump in Europe, while there is a separate study using a Dana pump taking place that aims to submit for FDA compliance in the US (currently discontinued due to Covid-19 situation). FDA approval submission is still part of the company’s development plans.
The data visuals from the Diabeloop system can be viewed on a cloud-based platform, called YourLoops, and shared with those in the user’s care group, including family members, partners or HCPs.
Diabeloop now has offices in Germany, a priority country for its plans. Earlier this year it also announced the conclusion of an agreement with global healthcare leader Terumo and a partnership with Roche Diabetes Care. Marc Julien, co-CEO of Diabeloop commented “We are very excited about these strong and reliable partnerships. They are key to expanding Diabeloop’s position in therapeutic artificial intelligence not just in France, but internationally – particularly in Europe.”